Introduction
The Barricaid Annular Closure Device is used to help prevent a recurrence of disc herniation following microdiscectomy surgery. Dr. Wang offers this state-of-the-art procedure to improve patient outcomes and ensure long-term results.
IS BARRICAID AN OPTION FOR ME?
Barricaid is meant for patients at higher risk of experiencing another disc herniation and return of back and leg pain.
Your spine surgeon will be able to determine, based on your pre-operative imaging scan, if you have a disc that can potentially be saved by performing a limited discectomy.
If you do, the hole in your disc will be measured during your discectomy surgery. If the hole is small you are considered at low risk of experiencing a repeat herniation and thus you will not need closure with the Barricaid implant.
However, when a large hole (≥6mm) is identified, you will benefit from closure with Barricaid.
Barricaid implantation immediately follows your standard discectomy procedure and the decision to utilize Barricaid is made intraoperatively by your surgeon.

BARRICAID SURGERY EXPLAINED
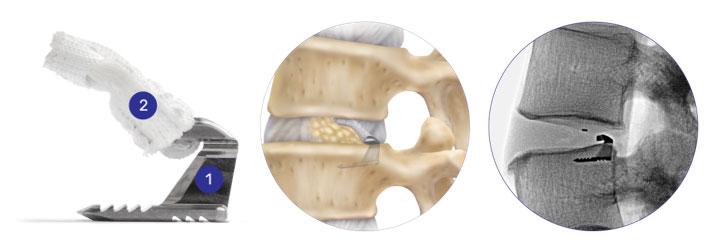
The Barricaid implant procedure is guided using x-ray. Your surgeon measures the size of the hole in your disc to see if you are at a higher risk of repeat herniation.
Your surgeon will then insert the small titanium anchor (1) into the bone, while the polyester (a type of plastic) flap (2) forms a barrier to block the hole in your disc.
The discectomy part of the surgery is supposed to reduce the pain and restore normal motion of your back. The Barricaid is designed to help reduce the potential for a future herniation to occur again.
CLINICAL RESULTS WITH BARRICAID
The Barricaid device has been implanted in over 7,500 patients worldwide since 2008. The device was rigorously tested in multiple clinical studies and approved for use by the U.S. FDA. A level I randomized controlled trial showed that the Barricaid reduced the risk for a reoperation caused by a repeat herniation by 60%, when compared to patients that did not receive the Barricaid implant1.

Risks
Standard discectomy surgery, with or without Barricaid, has some risks. It is important that you discuss all of the risks and benefits with your surgeon. Also see for complete SAFETY INFORMATION on Barricaid:

